Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 by Knudsen effusion mass spectrometry
by Knudsen effusion mass spectrometryNakajima, Kunihisa; Takai, Toshihide; Furukawa, Tomohiro; Osaka, Masahiko
Journal of Nuclear Materials, 491, p.183 - 189, 2017/08
Times Cited Count:8 Percentile:61.27(Materials Science, Multidisciplinary)One of the main chemical forms of cesium in the gas phase during severe accidents of light water reactor is expected to be cesium metaborate, CsBO , by thermodynamic equilibrium calculation considering reaction with boron. But accuracy of the thermodynamic data of gaseous metaborate, CsBO
, by thermodynamic equilibrium calculation considering reaction with boron. But accuracy of the thermodynamic data of gaseous metaborate, CsBO (g), has been judged as poor quality. Thus, Knudsen effusion mass spectrometric measurement of CsBO
(g), has been judged as poor quality. Thus, Knudsen effusion mass spectrometric measurement of CsBO was carried out to obtain reliable thermodynamic data. The evaluated values of standard enthalpy of formation of CsBO
was carried out to obtain reliable thermodynamic data. The evaluated values of standard enthalpy of formation of CsBO (g),
(g), 
 H
H
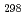 (CsBO
(CsBO ,g), by the 2nd and 3rd law treatments are -700.7
,g), by the 2nd and 3rd law treatments are -700.7 10.7 kJ/mol and -697.0
10.7 kJ/mol and -697.0 10.6 kJ/mol, respectively, and agree with each other within the errors, which suggests our data are reliable. Further, it was found that the existing data of the Gibbs energy function and the standard enthalpy of formation agreed well with the values evaluated in this study, which indicates the existing thermodynamic data are also reliable.
10.6 kJ/mol, respectively, and agree with each other within the errors, which suggests our data are reliable. Further, it was found that the existing data of the Gibbs energy function and the standard enthalpy of formation agreed well with the values evaluated in this study, which indicates the existing thermodynamic data are also reliable.
Ikusawa, Yoshihisa; Maeda, Koji; Kato, Masato; Uno, Masayoshi*
Nuclear Technology, 199(1), p.83 - 95, 2017/07
Times Cited Count:4 Percentile:37.06(Nuclear Science & Technology)Based on thermal computation results obtained using an irradiation behavior analysis code, we have evaluated the effect of O/M ratio on fuel restructuring from the results of PIEs for the B14 irradiation test fuel, which was a mixed oxide fuel and was irradiated in the experimental reactor Joyo. The thermal computation results showed that fuel restructuring in the stoichiometric oxide fuel was accelerated, though the fuel temperature in the stoichiometric oxide fuel was evaluated as lower than that of the hypo-stoichiometric one. We explained this behavior as follows: first, the fuel temperature decreased due to the high thermal conductivity at stoichiometry; second, the pore migration velocity increased due to the increase in vapor pressure caused by the high vapor pressure of UO , which was derived from the high oxygen potential at stoichiometry. In addition, our results indicated that the central void diameter strongly depended on not only fuel temperature, but also vapor pressure.
, which was derived from the high oxygen potential at stoichiometry. In addition, our results indicated that the central void diameter strongly depended on not only fuel temperature, but also vapor pressure.
 TiO
TiO heated at high temperature under various conditions
heated at high temperature under various conditionsHoshino, Tsuyoshi; Yasumoto, Masaru*; Tsuchiya, Kunihiko; Hayashi, Kimio; Nishimura, Hidetoshi*; Suzuki, Akihiro*; Terai, Takayuki*
Fusion Engineering and Design, 81(1-7), p.555 - 559, 2006/02
Times Cited Count:18 Percentile:76.2(Nuclear Science & Technology)no abstracts in English
Yamawaki, Michio*; Yamaguchi, Kenji; Suzuki, Atsushi*
Ionics, 7(4-6), p.339 - 345, 2001/07
Times Cited Count:6 Percentile:37.52(Chemistry, Physical)no abstracts in English
;
JNC TN9400 2000-034, 48 Pages, 2000/03
The study and the development to put FBR (Fast Breeder Reactor) to practical use have been doing. So many kinds of technologies are investigated to construct nuclear fuel recycle received to the society. The most important aim of reprocessing has been to extract U and Pu from spent fuels effectively, but, now, the demands for reprocessing are many kinds on nuclear fuel recycle system's construction. These need to be accepted sufficiently. The system that consists of electrolysis, extraction, with molten salt and melting metal, volatilization and condensation using the difference of vapor pressure is suggested, because, differently from LWR (Light Water Reactor), FBR can use the low decontamination factor's fuel. When the engineering scale plant is designed, the dry reprocessing has unsolved problems(ex. process flow) because of less demonstrative scale plants of the dry reprocessing than ones of the wet reprocessing. So the analysis and the estimation of mass balance that is most fundamental in the dry reprocessing system's design need to keep up with the system's alteration (to add new processes etc.) flexibly. This study aim is to develop the mass balance estimation code of dry reprocessing that satisfies the demand mentioned above.
Yamawaki, Michio*; Yamaguchi, Kenji*; Ono, Futaba*; Huang, J.*; Harada, Yuhei; Hidaka, Akihide; Sugimoto, Jun
JAERI-Tech 2000-015, p.38 - 0, 2000/03
no abstracts in English
 and BaUO
and BaUO in atmospheres simulating accident conditions
in atmospheres simulating accident conditionsHuang, J.*; *; Yamaguchi, Kenji*; *; *;
Journal of Nuclear Materials, 248, p.257 - 261, 1997/00
Times Cited Count:6 Percentile:47.96(Materials Science, Multidisciplinary)no abstracts in English
Arai, Yasuo; Nakajima, Kunihisa;
Proc. of 4th Int. Information Exchange Meeting on Actinide and Fission Product Partitioning and Transm, 0, p.347 - 357, 1997/00
no abstracts in English
 -KCl equimolar melt (Ln=Nd,Er) at high temperatures
-KCl equimolar melt (Ln=Nd,Er) at high temperatures*; *; Hashimoto, Masashi; Kudo, Hiroshi
Bulletin of the Chemical Society of Japan, 69(2), p.353 - 357, 1996/00
Times Cited Count:19 Percentile:68(Chemistry, Multidisciplinary)no abstracts in English
Yokota, Wataru; Nara, Takayuki; Arakawa, Kazuo; *; *
Nuclear Instruments and Methods in Physics Research B, 122(1), p.141 - 148, 1996/00
Times Cited Count:2 Percentile:30.98(Instruments & Instrumentation)no abstracts in English
Arai, Yasuo; ; Handa, Nuneo
Global 1995, Int. Conf. on Evaluation of Emerging Nuclear Fuel Cycle Systems,Vol. 1, 0, p.538 - 545, 1995/00
no abstracts in English
Ogawa, Toru
Journal of Alloys and Compounds, 203, p.221 - 227, 1994/00
Times Cited Count:8 Percentile:57.78(Chemistry, Physical)no abstracts in English
Maeda, Atsushi; ; Okamoto, Yoshihiro; Omichi, Toshihiko
Journal of Alloys and Compounds, 205, p.35 - 38, 1994/00
Times Cited Count:12 Percentile:66.82(Chemistry, Physical)no abstracts in English
 (M=Fe.Co,Ni)
(M=Fe.Co,Ni); ; ;
Journal of Nuclear Materials, 115, p.187 - 191, 1983/00
Times Cited Count:0 Percentile:0.02(Materials Science, Multidisciplinary)no abstracts in English
; Konishi, Satoshi; ; Kurasawa, T.; ; Katsuta, Hiroji; ; Naruse, Yuji
JAERI-M 82-194, 47 Pages, 1982/12
no abstracts in English

;
J.Phys.Chem., 82(7), p.780 - 784, 1978/07
no abstracts in English
;
JAERI-M 5946, 24 Pages, 1975/01
no abstracts in English
Takai, Toshihide; Nakajima, Kunihisa; Furukawa, Tomohiro; Osaka, Masahiko
no journal, ,
To develop reliable thermodynamic database of fission products is necessary for more accurate evaluation of fission product release and transport behavior using progression process analysis code under severe accident. This presentation introduces maintenance situation of high temperature mass spectrometer and reliability evaluation result.
Takai, Toshihide; Nakajima, Kunihisa; Furukawa, Tomohiro; Osaka, Masahiko
no journal, ,
Improvement of the serve accident analysis code is important to evaluate the distribution of the released fission products (FPs) in the reactor more precisely from the view point of both the removal preparation of the fuel debris in the Fukushima Daiichi Nuclear Power Station and improved source term evaluation. The development of the calculation model, which can evaluate the effect of atmosphere and the chemical reaction between the released FPs and control rod material (B C) at elevated temperature, is important for this purpose. The measurement technique of the equilibrium vapor pressure using a high temperature mass spectrometer was under development to expand the thermodynamic database of the simulated FPs, because the chemical equilibrium analysis plays an important role to clarify the effect of chemical reactions. In this study, the apparatus was developed for the equilibrium vapor pressure measurements, and then the reliability of measurement results was evaluated.
C) at elevated temperature, is important for this purpose. The measurement technique of the equilibrium vapor pressure using a high temperature mass spectrometer was under development to expand the thermodynamic database of the simulated FPs, because the chemical equilibrium analysis plays an important role to clarify the effect of chemical reactions. In this study, the apparatus was developed for the equilibrium vapor pressure measurements, and then the reliability of measurement results was evaluated.
Sekiguchi, Yuma*; Terai, Takayuki*; Uozumi, Koichi*; Koyama, Tadafumi*; Amamoto, Ippei
no journal, ,
no abstracts in English